Chemistry Nomenclature Chart
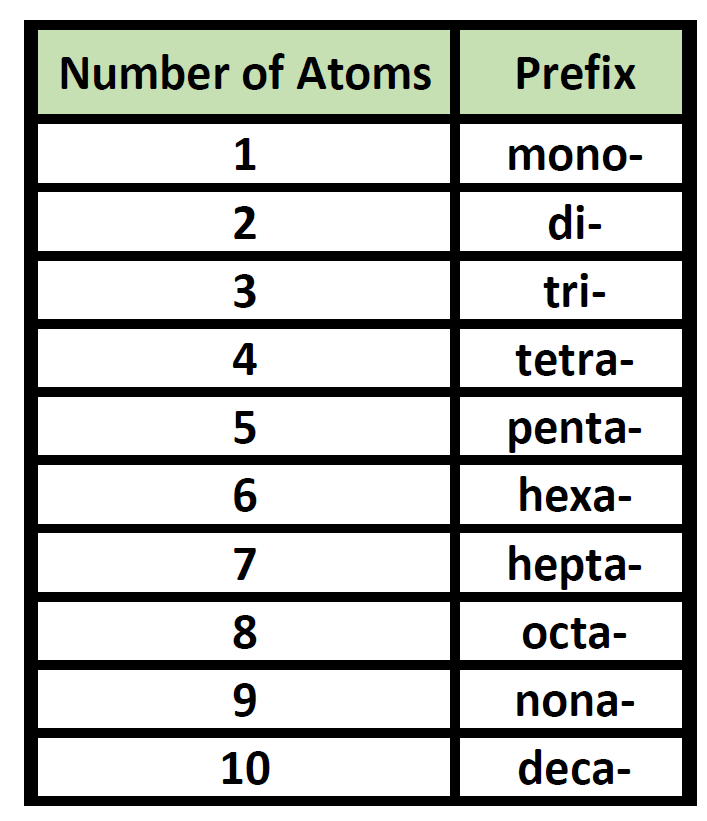
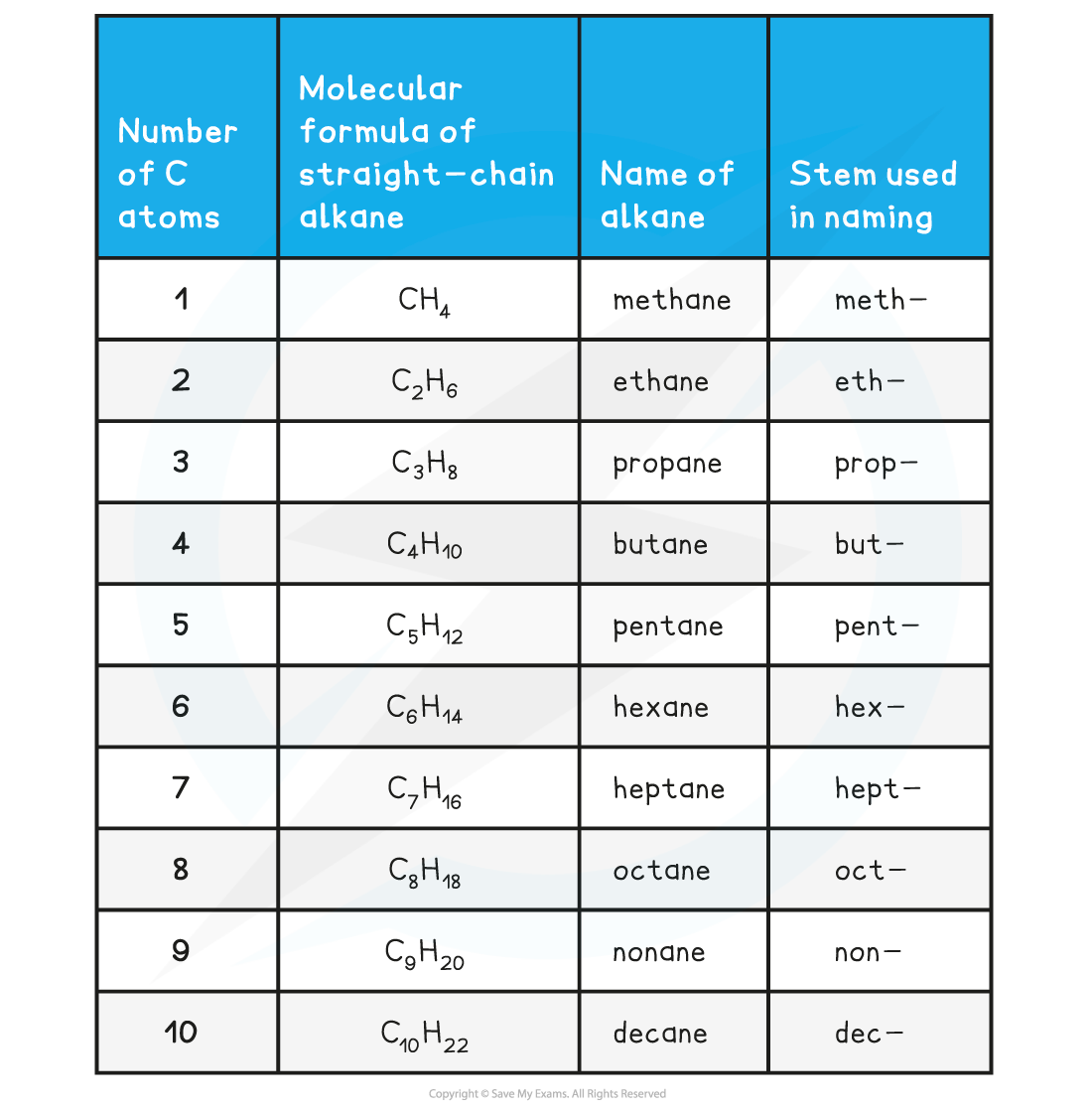
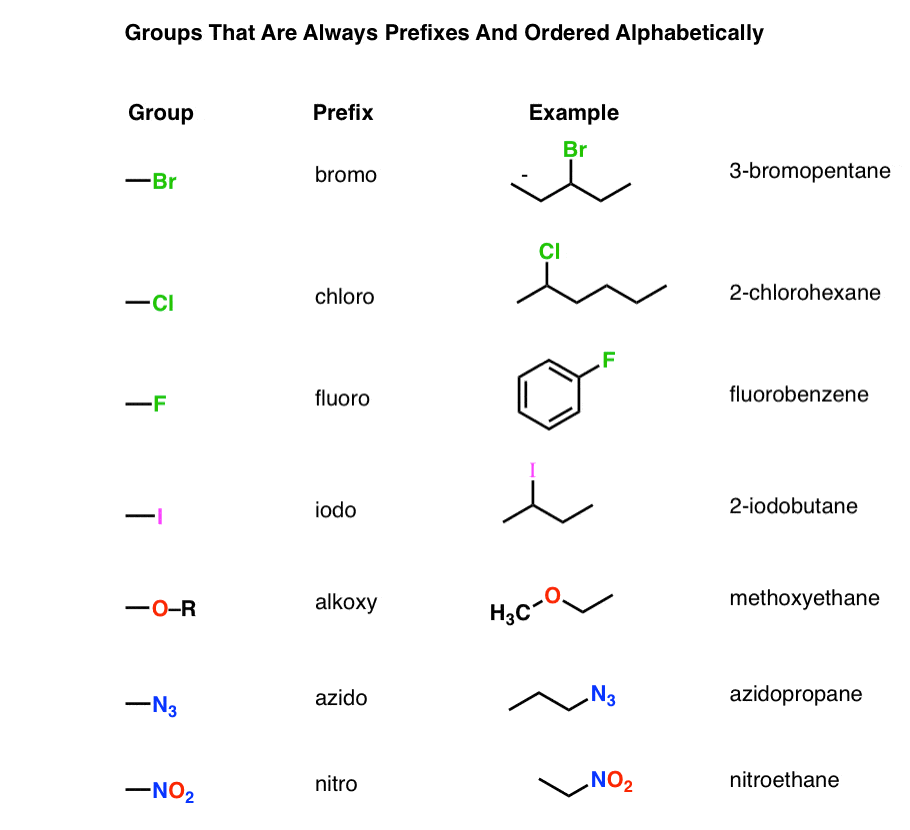
Chemistry Nomenclature Chart - Web table 2.7.1 2.7. Introduction to chemical nomenclature is shared under a cc by 3.0 license and was authored, remixed, and/or curated by stephen lower via source content that was edited to the style and standards of the libretexts platform. Chemical reactivity and products of chemical reactions; Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). Web nomenclature, a collection of rules for naming things, is important in science and in many other situations. Relationships in the periodic table: There are several general rules, however, that can bring some order out of this apparent chaos. Common greek prefixes used in naming simple moleculs. The nomenclature used most frequently worldwide is the one created and developed by the international union of pure and applied chemistry (iupac). Elements combine to form compounds. Examples of this include phenol, acetic acid, and toluene. There are several general rules, however, that can bring some order out of this apparent chaos. Chemistry of the main groups and transition elements, including typical. This module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). Nomenclature, a collection of rules for naming things, is important in science and in many other situations. Because the elements are chemically combined, they cannot be separated using physical means. There are some rules we need to follow,which are: There does not exist any particular collection of rules for writing the trivial naming of compounds. The basics of organic nomenclature, of inorganic nomenclature and polymer nomenclature are summarized in a collection of brief guides accessible below. Chemical reactivity and products of chemical reactions; Examples of this include phenol, acetic acid, and toluene. In addition, the properties of the compound (both physical and chemical) are radically. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. Elements combine to form compounds. If there are two atoms, place the more metallic first (furthest to the left on the periodic table) is there is a central atom, place it first, and the atoms attached after. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. Web develop an understanding of systematic nomenclature that facilitates communication; Common. Acid usually means an anion combined with the hydrogen ion as the cation. Relationships in the periodic table: Web chemical nomenclature for acids. Iupac nomenclature is used for the naming of chemical compounds, based on their chemical composition and their structure. As one of its major activities, iupac. Chemical nomenclature is a set of rules to generate systematic names for chemical compounds. Web derive names for common types of inorganic compounds using a systematic approach. This module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. Examples of this include phenol, acetic acid, and. If you have a molecule with, say, a carboxylic acid and a ketone you consult the table. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. Iupac nomenclature ensures that each compound (and its various isomers). Web chemical nomenclature for acids. Nomenclature, a collection of rules for naming things, is important in. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. In addition, the properties of the compound (both physical and chemical) are radically. Web develop an understanding of systematic nomenclature that facilitates communication; As one of its major activities, iupac. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. If you have a molecule with, say, a carboxylic acid and a ketone you consult the table. As one of its major activities, iupac. Chemistry of. Iupac nomenclature ensures that each compound (and its various isomers). Web at first glance, the nomenclature of the polyatomic negative ions in the table above seems hopeless. Introduction to chemical nomenclature is shared under a cc by 3.0 license and was authored, remixed, and/or curated by stephen lower via source content that was edited to the style and standards of. If there are two atoms, place the more metallic first (furthest to the left on the periodic table) is there is a central atom, place it first, and the atoms attached after. This module describes an approach that is used to name simple ionic and molecular compounds, such as nacl, caco 3, and n 2 o 4. For instance, hcl. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. Common greek prefixes used in naming simple moleculs. Web develop an understanding of systematic nomenclature that facilitates communication; There are several general rules, however, that can bring some order out of this apparent chaos. Bonding, werner’s theory, vbt, and cft; Web derive names for common types of inorganic compounds using a systematic approach. There does not exist any particular collection of rules for writing the trivial naming of compounds. The simplest of these are binary compounds, those containing only two elements, but we will also consider how. Common greek prefixes used in naming simple moleculs. As one of its major activities, iupac. If there are two atoms, place the more metallic first (furthest to the left on the periodic table) is there is a central atom, place it first, and the atoms attached after. A compound is a substance that contains two or more elements chemically combined in a fixed proportion. Acid usually means an anion combined with the hydrogen ion as the cation. Chemical nomenclature is far too big a topic to treat. Web in chemical nomenclature, the iupac nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended [1] [2] by the international union of pure and applied chemistry (iupac). Web iupac provides recommendations on many aspects of nomenclature. Relationships in the periodic table: Chemical reactivity and products of chemical reactions; For instance, hcl is a common. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. And so, iupac (think of the “ministry of magic”, but for chemists) has developed one.NEET Chemistry Nomenclature of Organic Compounds IUPAC Rules YouTube
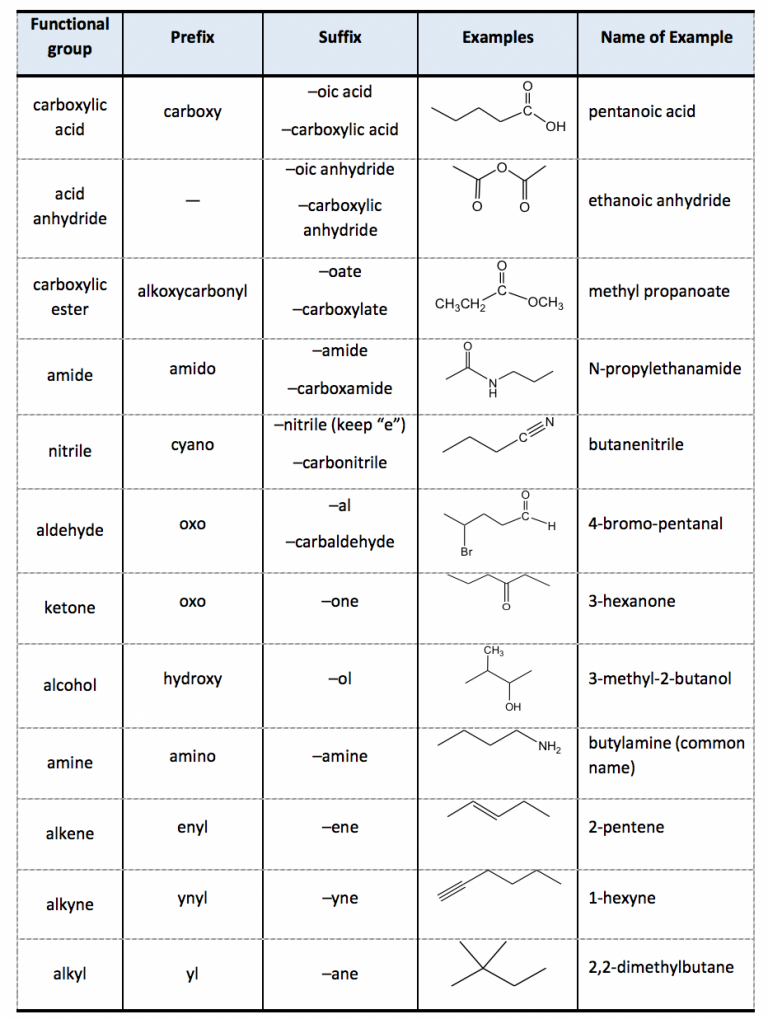
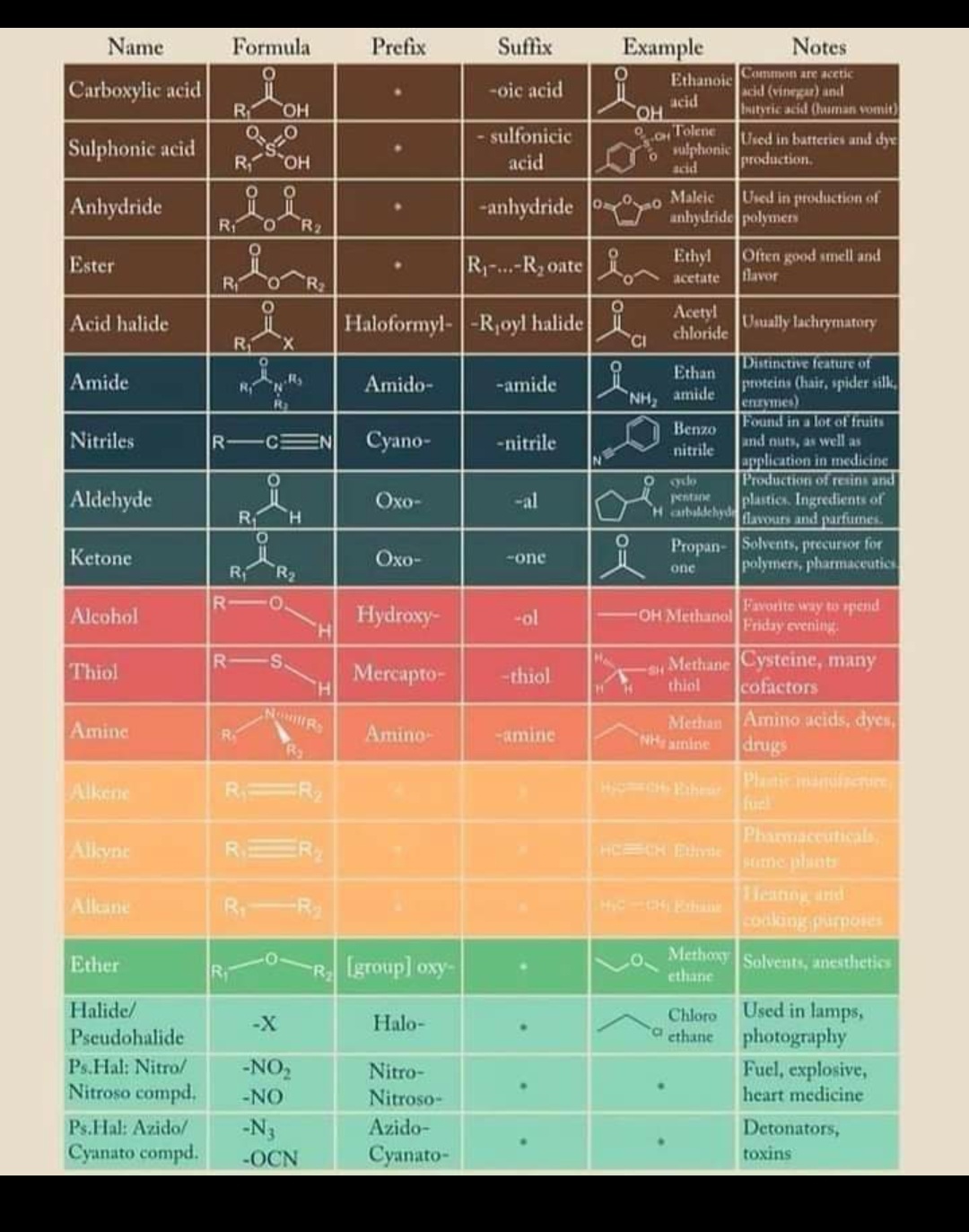
Table of Functional Group Priorities for Nomenclature Master Organic
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
IB DP Chemistry HL复习笔记10.1.3 Nomenclature翰林国际教育
Table of Functional Group Priorities for Nomenclature Master Organic
Organic Chemistry Nomenclature Cheat Sheet Cheat Sheet
Nomenclature of Acids Pathways to Chemistry
07 Nomenclature Mrs. Cook's Chemistry Class
Chemistry Nomenclature Chart
Chemistry Chart
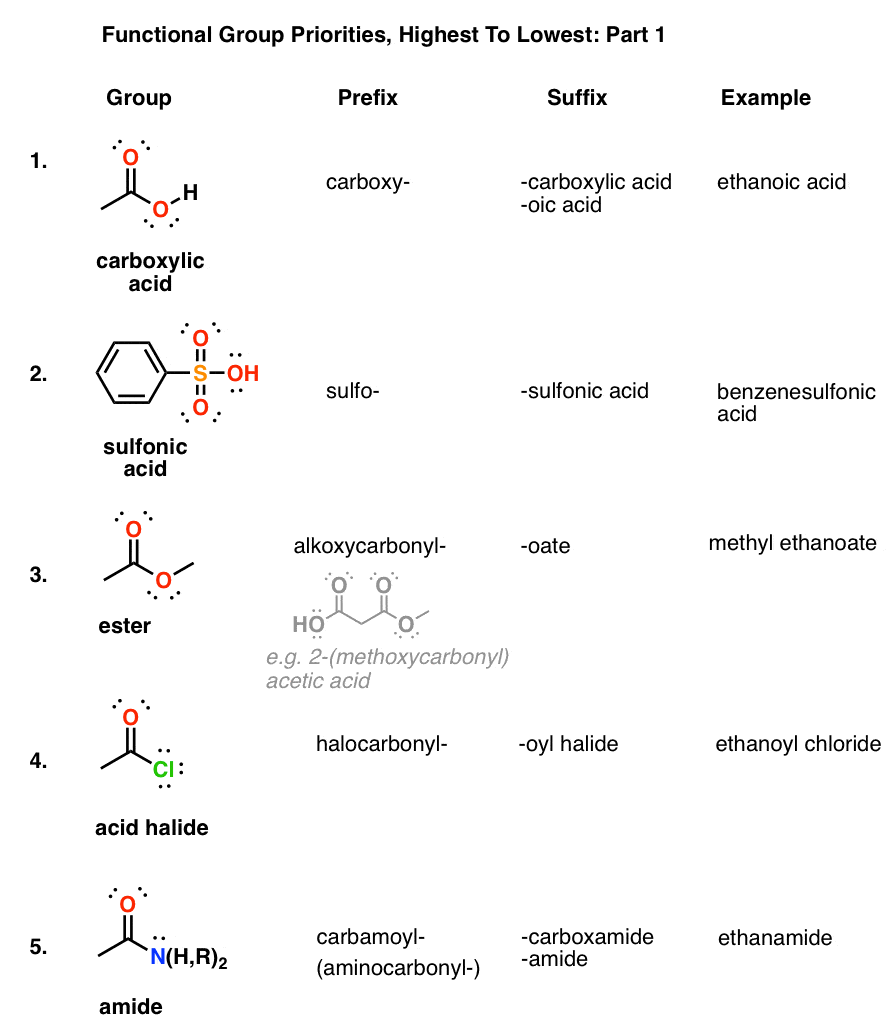
Web We Need Some Kind Of Priority System For Nomenclature.
Web Table 2.7.1 2.7.
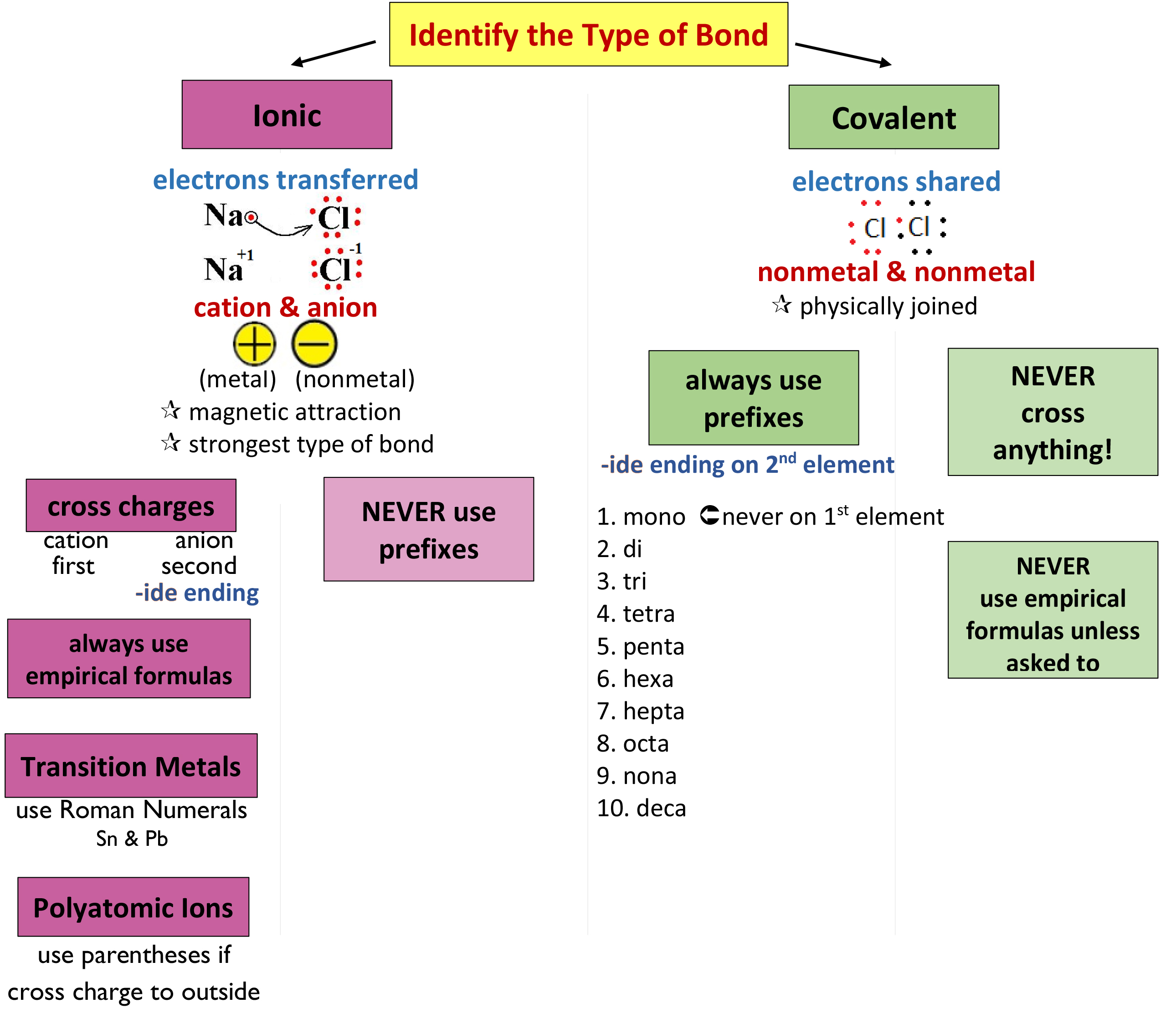
This Module Describes An Approach That Is Used To Name Simple Ionic And Molecular Compounds, Such As Nacl, Caco 3, And N 2 O 4.
There Are Some Rules We Need To Follow,Which Are:
Related Post: