Bond Energies Chart
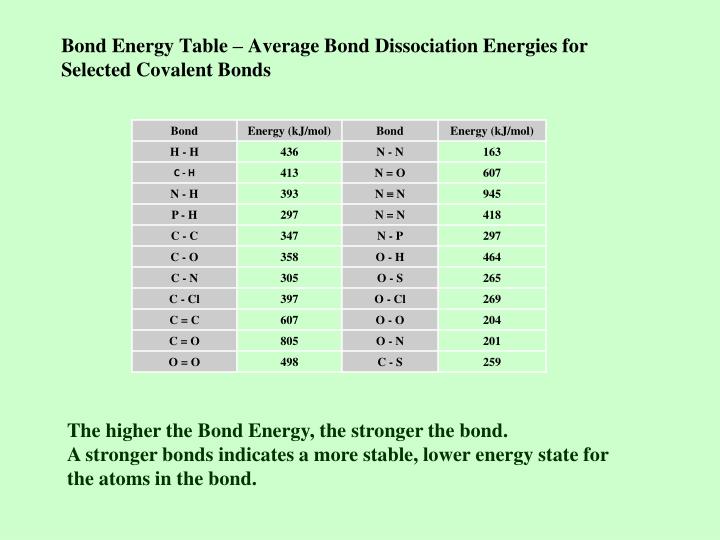
Bond Energies Chart - Cottrell, the strengths of chemical bonds, 2nd ed., butterworths, london, 1958; Bond energies are also called bond enthalpies, and in the past have been known as bond strengths. Bond order is the number of electron pairs that hold two atoms together. Explain its relationship to bond length or bond energy. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). We often use a more condensed form of bond energy tables as shown below. In proposing his theory that octets can be completed by two atoms sharing electron pairs, lewis provided scientists with the first description of covalent bonding. The si units used to describe bond energy is kilojoules per mole of bonds (kj/mol). So, ‘’the bond energy is the average amount of energy required to break all bonds of a particular type in one mole of the substance’’. Web average bond energies *bond dissociation energies for diatomic molecules can be directly measured. Cottrell, the strengths of chemical bonds, 2nd ed., butterworths, london, 1958; We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to get the average bond energy. (assume complete combustion) check solutions/answers to exercises. Web this equation can solve for any of these three values and is a key formula in thermodyanamics, physical chemistry, and more. Web this page has tables of standard bond energies and bond dissociation energies. Web properties of atoms, radicals, and bonds 4.41 table 4.11 bond dissociation energies the bond dissociation energy (enthalpy change) for a bond a 9b which is broken through the reaction ab : To define and used average bond energies. Bond strength qualities and periodic trends. The si units used to describe bond energy is kilojoules per mole of bonds (kj/mol). You can take all these terms as meaning the same thing. The following tables list experimental bond dissociation enthalpies of common bonds at 298 k. Cottrell, the strengths of chemical bonds, 2nd ed., butterworths, london, 1958; Web atoms are held together by a certain amount of energy called bond energy. That is, hf 298 298 298 298 hf (a). 2 nh 3 (g) + cl 2 (g) → n 2 h. When a bond is formed between two atoms, energy is released. We often use a more condensed form of bond energy tables as shown below. Web table shows energy of common chemical bonds in selected unit (kj/mol, atomic units, ev etc.). The same amount of energy is absorbed when the bond is broken to form neutral atoms. Values are in. Explain its relationship to bond length or bond energy. Bond enthalpies are essentially a measure of how strong a covalent bond is. * average bond dissociation enthalpies in kcal per mole (there can be considerable variability in some of these values.) Most commonly, a bond’s strength depends on: So, ‘’the bond energy is the average amount of energy required to. Web the bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. Values are in kj/mol of bonds. When a bond is formed between two atoms, energy is released. Bond strength qualities and periodic trends. So, ‘’the bond energy is the average amount of energy required to break all bonds. (assume complete combustion) check solutions/answers to exercises. Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively. The following tables list experimental bond dissociation enthalpies of common bonds at 298 k. Explain its relationship to bond length or bond energy. Web atoms are held together by a certain amount of energy called. Web the energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. This page introduces bond energies and looks at how they can be used to estimate the enthalpy change for some simple reactions. Use the bond energies from the table to calculate an approximate δh. Explain its relationship to bond length or bond energy. * average bond dissociation enthalpies in kcal per mole (there can be considerable variability in some of these values.) The rest are average bond energies. We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to get the. The rest are average bond energies. Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively. We often use a more condensed form of bond energy tables as shown below. Bond enthalpies are essentially a measure of how strong a covalent bond is. Web average bond energies *bond dissociation energies for diatomic. This is typically the types of tables that are put on exams to save space. This page introduces bond energies and looks at how they can be used to estimate the enthalpy change for some simple reactions. Bond order is the number of electron pairs that hold two atoms together. Web bond s— s— f cl energy 218 212 215. Web atoms are held together by a certain amount of energy called bond energy. Explain its relationship to bond length or bond energy. Use the bond energies from the table to calculate an approximate δh 0rxn for the following equation: To define and used average bond energies. Bond order is the number of electron pairs that hold two atoms together. * average bond dissociation enthalpies in kcal per mole (there can be considerable variability in some of these values.) Web properties of atoms, radicals, and bonds 4.41 table 4.11 bond dissociation energies the bond dissociation energy (enthalpy change) for a bond a 9b which is broken through the reaction ab : To define and used average bond energies. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). Recognize covalent substances and characterize ionic character as difference in electronegativity. Bond enthalpies are essentially a measure of how strong a covalent bond is. Web this page has tables of standard bond energies and bond dissociation energies. The si units used to describe bond energy is kilojoules per mole of bonds (kj/mol). You can take all these terms as meaning the same thing. The same amount of energy is absorbed when the bond is broken to form neutral atoms. That is, hf 298 298 298 298 hf (a). This is typically the types of tables that are put on exams to save space. We often use a more condensed form of bond energy tables as shown below. When a bond is formed between two atoms, energy is released. Web the average bond energy is therefore +1662/4 kj, which is +415.5 kj per mole of bonds. So, ‘’the bond energy is the average amount of energy required to break all bonds of a particular type in one mole of the substance’’.Bond Length and Bond Strength Pathways to Chemistry
Bond Energies Chart
Table Of Bond Energies Pathways To Chemistry vrogue.co
Bond Length and Bond Energy
Bond Energy Profile Chart
The Heat of Reaction from Bond Dissociation Energies Chemistry Steps
Bond Energies Chart
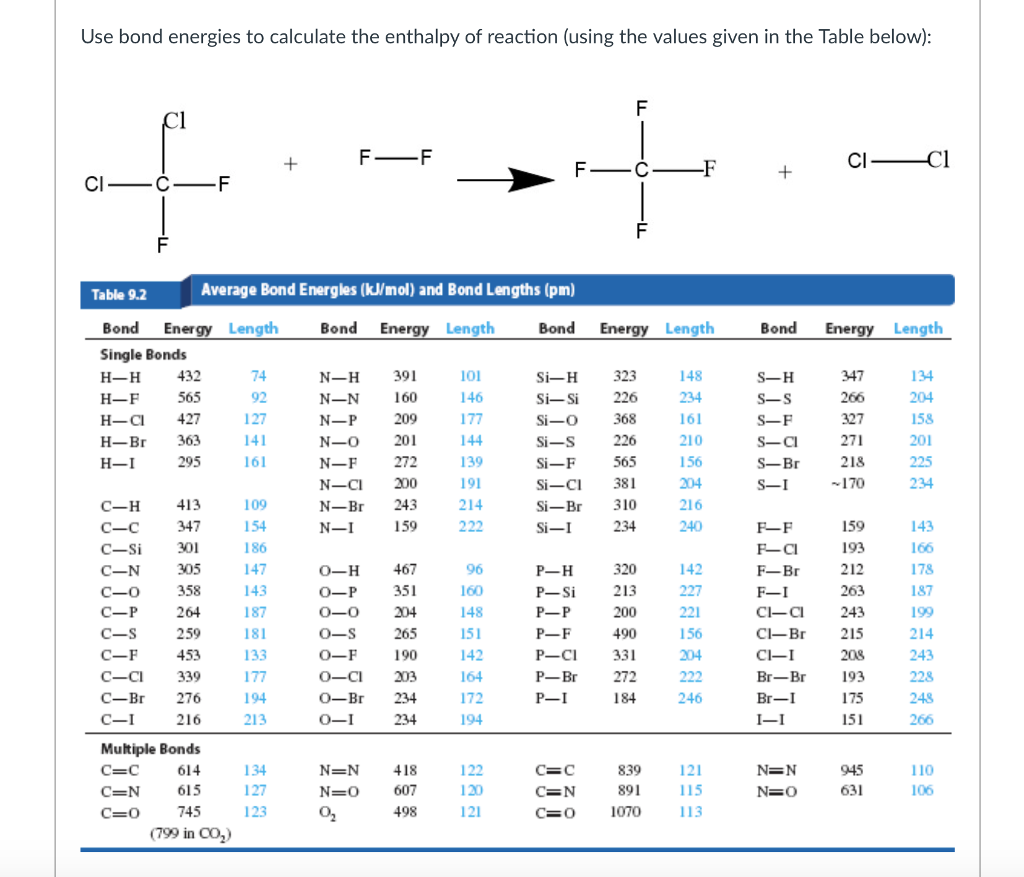
[Solved] Using the appropriate bond energies, calculate the heat of
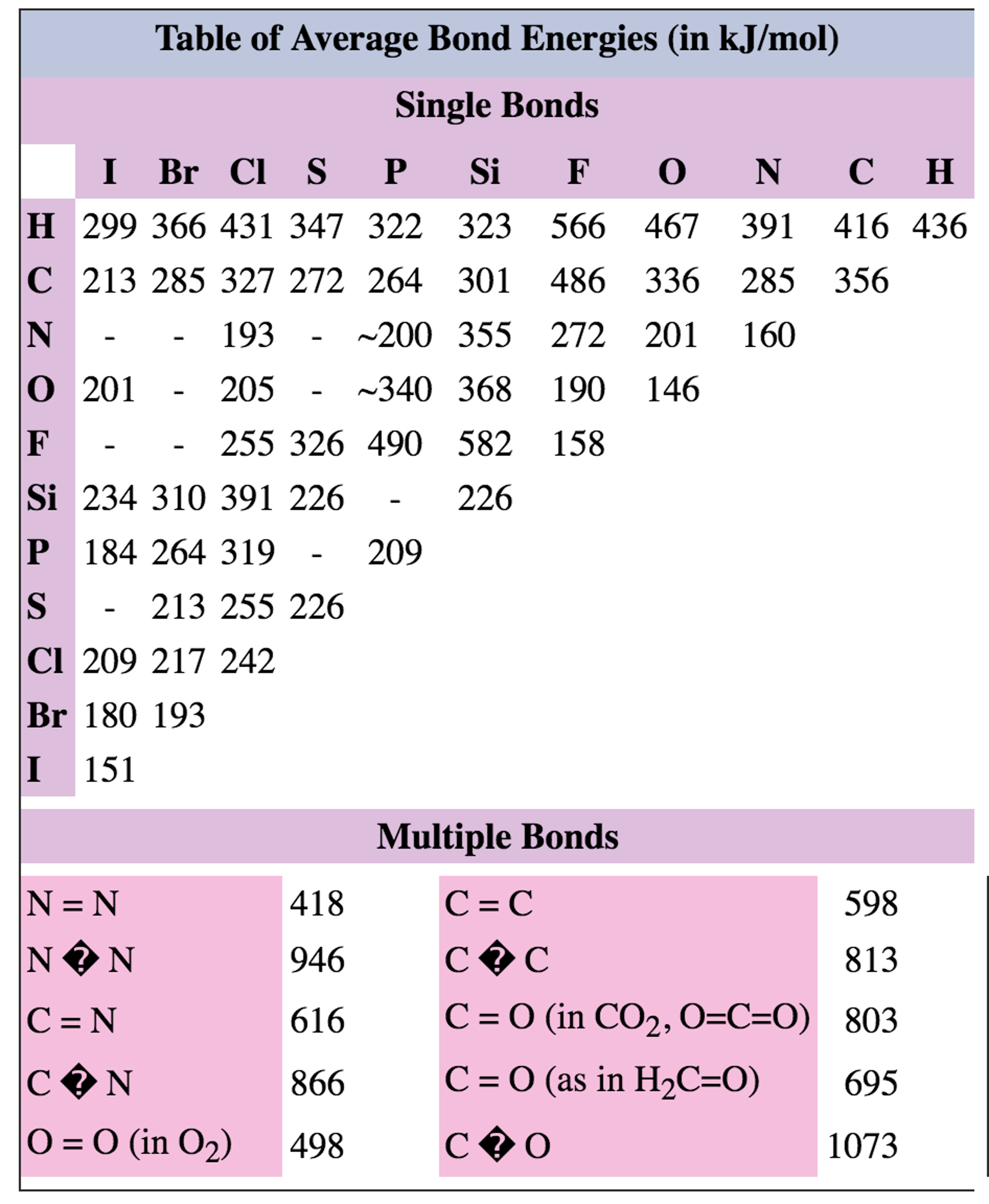
Average Bond Energies Chart
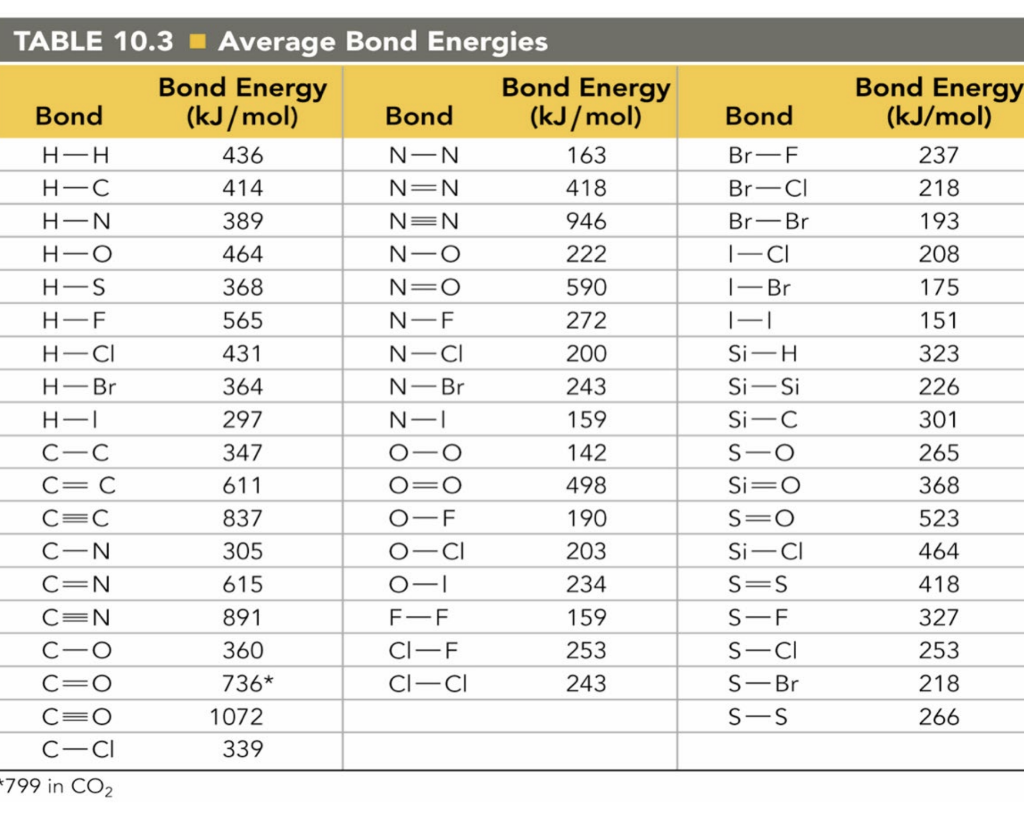
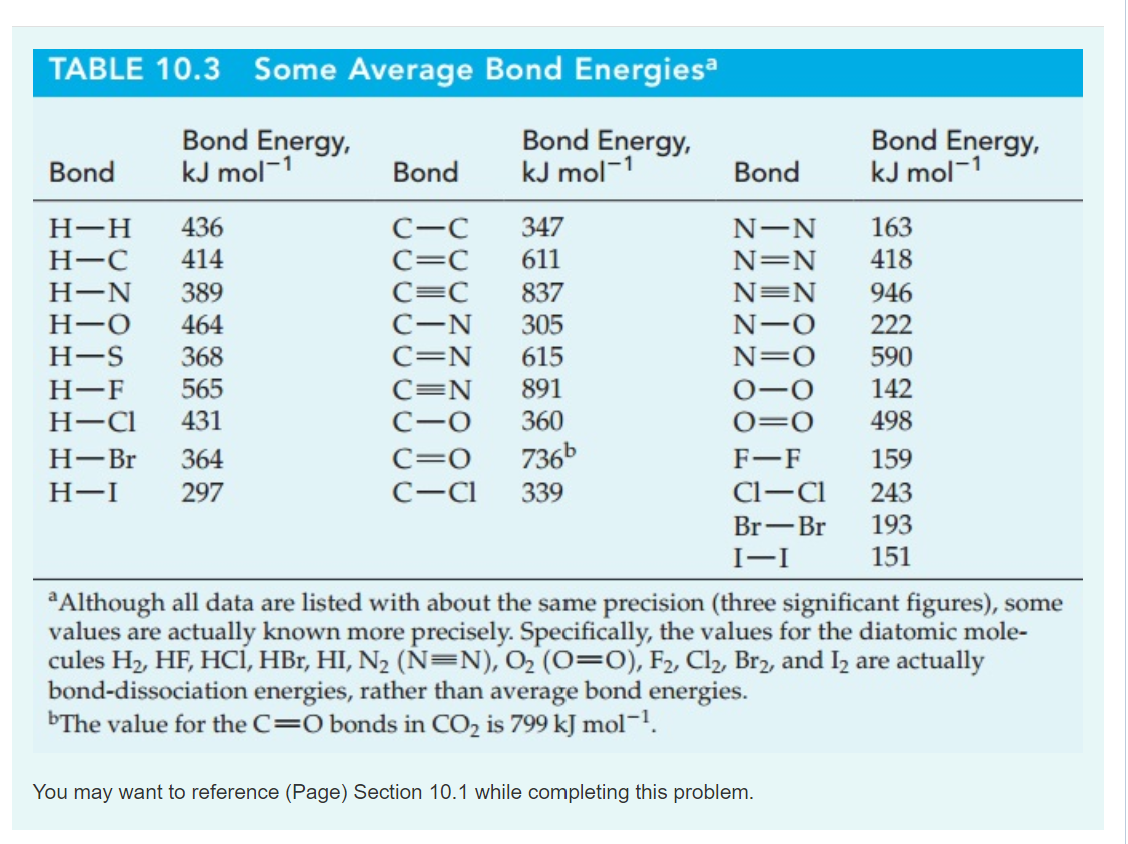
Solved Bond TABLE 10.3 Average Bond Energies Bond Energy
That Means That Many Bond Enthalpies Are Actually Quoted As Mean (Or Average) Bond Enthalpies, Although It Might Not Actually Say So.
This Page Introduces Bond Energies And Looks At How They Can Be Used To Estimate The Enthalpy Change For Some Simple Reactions.
Web Bond S— S— F Cl Energy 218 212 215 Bond Energy 432 427 363 347 305 358 453 339 276 216 614 (799 In C02) Single Bonds H— H— H— C— C— C— C— C— C C— C— C C— H C Si N O S Multiple Bonds C N O.
Web Table Shows Energy Of Common Chemical Bonds In Selected Unit (Kj/Mol, Atomic Units, Ev Etc.).
Related Post:

.PNG)